Background:
Bronchiolitis obliterans syndrome (BOS) occurring after allogeneic HCT is a pulmonary manifestation of chronic GVHD (cGVHD) that is associated with poor outcomes. Ruxolitinib, an oral selective JAK1/2 inhibitor, is approved for the treatment of recurrent/refractory cGVHD. However, efficacy of ruxolitinib for BOS is not well-characterized.
Methods:
In this prospective, multicenter phase II trial (NCT03674047), adult subjects (age 18-75) with BOS were enrolled at 7 academic centers. BOS was defined by either NIH criteria (Jagasia et al. BBMT. 2015) or atypical (Bergeron et al. BMT. 2013) criteria. Subjects were ≥4 weeks since start of most recent systemic GVHD therapy. Subjects were enrolled into a newly diagnosed cohort (<6 mo since BOS diagnosis, cohort A) or an established BOS cohort (≥6 mo since BOS diagnosis, cohort B), and were treated with ruxolitinib 10 mg BID continuously in 28-d cycles, for up to 12 cycles. A treatment extension up to 36 cycles was allowed for subjects benefiting from treatment. The primary objective was to evaluate the early treatment effect of ruxolitinib on BOS, assessed by % change in FEV1 at 3 months compared to baseline. Secondary objectives included NIH lung-specific ORR, treatment-emergent toxicities and infection, longitudinal changes in corticosteroid dosing, patient-reported outcomes, and exploratory evaluation of GVHD and BOS-associated cytokines.
Results:
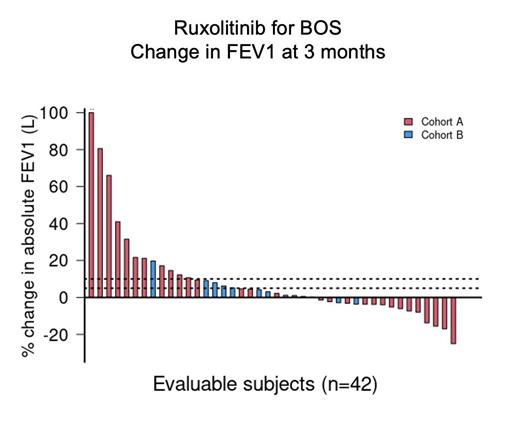
Fifty subjects were enrolled (cohort A, n=37; cohort B, n=13). The median age for the entire cohort was 62 years (range, 21-71). At enrollment, the NIH lung score based on %FEV1 of predicted were 1 (n=19, 38%), 2 (n=23, 46%) or 3 (n=8, 16%). 33 subjects met NIH diagnostic criteria for BOS. At enrollment, 37 subjects were being treated with prednisone (median dose: 20 mg/d). The median follow-up of survivors is 19 months (range, 2-31) at last cutoff (3/31/23). The median number of cycles of treatment is 10 (range, 1-12; treatment extension not included in current analysis). 17 subjects remain on therapy; reasons for discontinuation include: completion therapy (n=7), BOS or GVHD progression (n=6), relapse (n=6), physician discretion (n=4), AEs (n=3), death (n=3), subject withdrawal (n=2), and lost follow-up (n=2). When comparing PFTs after 3 cycles of ruxolitinib to baseline, newly diagnosed BOS (cohort A) experienced more dynamic early changes in FEV1 (11 subjects with ≥10% increase, 8 subjects with ≥5% decrease); in established BOS (cohort B), disease was stable (1 subject with ≥10% increase, no subjects with ≥5% decrease) ( Figure 1). According to the NIH response criteria, the best lung-specific ORR for the entire study is 34% (18% CR, 16% PR). ORR did not differ between cohorts (cohort A: 36%, cohort B: 31%; p=1.0) nor BOS classification (NIH: 29%, atypical: 44%; p=0.2). ORR according to the baseline disease was 44%, 16% and 57% for NIH lung scores of 1, 2, and 3, respectively (p=0.07). In univariate analysis, only the total number of GVHD sites involved at baseline was significantly associated with lung-specific response (OR 4.7 for <3 vs ≥3, 95%CI 1.23-18, p=0.023); however, this did not meet criteria for significance in multivariate analysis (OR 6.3, p=0.06). The most common non-infectious severe (grade ≥3) TEAEs were neutropenia (n=3), anemia (n=2), and hypertension (n=2). There were 8 grade ≥3 infectious events: upper respiratory infection (n=6) and pneumonia (n=2). No fungal infections were observed. The median reduction in steroid dose was 50% by the end of treatment and 14% of subjects were able to discontinue steroids altogether. Clinically meaningful improvement (≥7-point reduction) in mLSS score on consecutive assessments was observed in 45% of subjects (responders: 50%; non-responders: 44%). However, only 3 subjects (7.5%) experienced a clinically meaningful organ-specific change (≥10-point reduction) in mLSS lung scores. The 1-year FFS and OS probabilities are 79% (95%CI, 64-88) and 89% (95%CI, 75-95), respectively. PRO and exploratory correlative studies are being analyzed.
Conclusion:
Ruxolitinib is associated with clinical responses in BOS, which were observed across all severity of disease. Ruxolitinib was associated with few severe AEs, including severe infections. Most subjects were able to taper or discontinue corticosteroids while on trial treatment. These results support the use of ruxolitinib in the management of BOS after allogeneic HCT.
Disclosures
Defilipp:Inhibrx: Consultancy; PharmaBiome AG: Consultancy; MorphoSys: Consultancy; Sanofi: Consultancy; Taiho Oncology: Research Funding; Regimmune: Research Funding; Incyte: Consultancy, Research Funding; Ono Pharmaceutical: Consultancy. Hamadani:AstraZeneca: Speakers Bureau; Legend Biotech: Consultancy; Kite, a Gilead Company: Consultancy, Speakers Bureau; BeiGene: Speakers Bureau; MorphoSys: Consultancy; Genentech: Honoraria; Takeda: Research Funding; Spectrum Pharmaceuticals: Research Funding; Astellas: Research Funding; BeiGene: Speakers Bureau; Sanofi Genzyme: Speakers Bureau; ADC therapeutics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Astra Zeneca: Speakers Bureau; Abbvie: Consultancy; Omeros: Consultancy; SeaGen: Consultancy; CRISPR: Consultancy; Genmab: Consultancy; Bristol Myers Squibb: Consultancy; Novartis: Consultancy; Caribou: Consultancy; Kadmon: Consultancy; Myeloid Therapeutics: Honoraria; Gamida Cell: Consultancy; Incyte: Consultancy; Genmab: Consultancy. Sandhu:Autolus Therapeutics: Consultancy; City of Hope Medical Center: Current Employment. Hamilton:Kadmon/Sanofi: Other: advisory board; Incyte: Other: ad hoc consultancy; Equilium: Other: ad hoc advisory board; NKARTA: Other: ad hoc advisory board; Angiocrine: Other: DSMB; Therakos: Honoraria; Rigel: Other: Ad hoc advisory board; CSL Behring: Other: Adjudication committee. Lee:Kadmon: Honoraria; Fresenius Kabi: Consultancy; BMS: Honoraria; Kite Pharma: Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria; Incyte Corp: Consultancy, Research Funding. El-Jawahri:GSK: Consultancy; Novartis: Consultancy; Incyte Corporation: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal